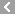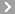CAS No.:15351-05-0
Name: Buzepide methiodide
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intramuscular | 201mg/kg (201mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR BEHAVIORAL: ATAXIA |
Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| mouse | LD50 | intraperitoneal | 94mg/kg (94mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| mouse | LD50 | intravenous | 14mg/kg (14mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| mouse | LD50 | oral | 820mg/kg (820mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS | Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| mouse | LD50 | subcutaneous | 229mg/kg (229mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| rat | LD50 | intramuscular | 800mg/kg (800mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA |
Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| rat | LD50 | intraperitoneal | 145mg/kg (145mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| rat | LD50 | intravenous | 29300ug/kg (29.3mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| rat | LD50 | oral | 2800mg/kg (2800mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS | Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
| rat | LD50 | subcutaneous | 1210mg/kg (1210mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Kiso to Rinsho. Clinical Report. Vol. 4, Pg. 1850, 1970. |
Consensus Reports:
SAFETY PROFILE:
A poison by intraperitoneal, intravenous, intramuscular, and subcutaneous routes. Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of I− and NOx. See also IODIDES.
Standards and Recommendations:
Analytical Methods:
Related Searches
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione