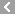CAS No.:26787-78-0
Name: Amoxicillin
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 300mg/kg (300mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: HEMATURIA KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION |
Clinical Pediatrics Vol. 32, Pg. 735, 1993. |
| child | TDLo | oral | 682mg/kg (682mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: URINE VOLUME DECREASED KIDNEY, URETER, AND BLADDER: HEMATURIA |
Clinical Pediatrics Vol. 32, Pg. 735, 1993. |
| man | TDLo | oral | 7143ug/kg (7.143mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Allergy. Vol. 52(Suppl, |
| man | TDLo | oral | 40mg/kg/4D (40mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" GASTROINTESTINAL: OTHER CHANGES |
Lancet. Vol. 2, Pg. 707, 1978. |
| mouse | LD50 | intracrebral | > 500mg/kg (500mg/kg) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS | Chemotherapy Vol. 26, Pg. 196, 1980. |
| mouse | LD50 | intraperitoneal | 3590mg/kg (3590mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| mouse | LD50 | oral | > 25gm/kg (25000mg/kg) | Drugs in Japan Vol. 6, Pg. 38, 1982. | |
| mouse | LD50 | subcutaneous | > 20mg/kg (20mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| rat | LD50 | intraperitoneal | 2870mg/kg (2870mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| rat | LD50 | oral | > 15gm/kg (15000mg/kg) | Drugs in Japan Vol. 6, Pg. 38, 1982. | |
| rat | LD50 | subcutaneous | > 8gm/kg (8000mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. |
Consensus Reports:
SAFETY PROFILE:
Hazard Codes :  Xn,
Xn, Xi
Xi
Risk Statements : 42/43
R42/43:May cause sensitization by inhalation and skin contact.
Safety Statements : 22-36/37
S22:Do not breathe dust.
S36/37:Wear suitable protective clothing and gloves.
WGK Germany : 2
RTECS : XH8300000
Standards and Recommendations:
Purity of amoxicillin: 98.00%
Analytical Methods:
Related Searches

Related Suppliers
- LIDE PHARMACEUTICALS LIMITED
- Afine Chemicals Limited
- Josun International Limited
- BOC Sciences
- SJZ THLD IMP & EXP CO LTD
- BHAGERIA DYE CHEM LIMITED
- Bristol Laboratories Ltd
- Improve Medical Technology(Nanxiong) Co., Ltd
- DC Fine Chemicals Ltd
- North China Pharmaceutical Group Corporation
- Anchor Chemicals, Inc.
- Beijing Boyakaihua Company
- Sagran
- Surya Pharmaceutical Limited
- Zhejiang Chemline International Co., Ltd.
- Wuhan Taiyicheng Biopharmaceutical Co., Ltd.,
- Jiangxi Dongxu Chemical Science & Technology Co.,Ltd
- LGM Pharma
- TOKU-E Company
- SEA BRGIHT INDUSTRY CO.,LIMITED
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione