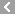CAS No.:12192-57-3
Name: Aurothioglucose
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| chicken | LD50 | intravenous | 1gm/kg (1000mg/kg) | Poultry Science. Vol. 52, Pg. 926, 1973. | |
| chicken | LDLo | intramuscular | 300mg/kg (300mg/kg) | BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES SPINAL CORD: OTHER DEGENERATIVE CHANGES |
Toxicology and Applied Pharmacology. Vol. 35, Pg. 223, 1976. |
| man | LDLo | unreported | 3mg/kg (3mg/kg) | Sammlung von Vergiftungsfaellen. Vol. 10, Pg. 101A, 1939. | |
| man | TDLo | intramuscular | 3357ug/kg/4W- (3.357mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE | Journal of Rheumatology. Vol. 12, Pg. 619, 1985. |
| man | TDLo | intramuscular | 5500ug/kg/10W (5.5mg/kg) | LUNGS, THORAX, OR RESPIRATION: COUGH LUNGS, THORAX, OR RESPIRATION: DYSPNEA |
Southern Medical Journal. Vol. 88, Pg. 644, 1995. |
| mouse | LD50 | intraperitoneal | 2gm/kg (2000mg/kg) | LIVER: OTHER CHANGES | Proceedings of the Society for Experimental Biology and Medicine. Vol. 70, Pg. 498, 1949. |
| mouse | LDLo | subcutaneous | 1650mg/kg (1650mg/kg) | Experimental Medicine and Surgery. Vol. 3, Pg. 146, 1945. | |
| women | TDLo | intramuscular | 2600ug/kg/15D (2.6mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING LIVER: OTHER CHANGES |
Arthritis and Rheumatism. Vol. 27, Pg. 230, 1984. |
| women | TDLo | intramuscular | 14402ug/kg/2Y (14.402mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: PROTEINURIS KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION |
Taisha. Molecular Medicine Vol. 12, Pg. 1435, 1975. |
| women | TDLo | parenteral | 2700ug/kg/4W- (2.7mg/kg) | LIVER: "JAUNDICE, CHOLESTATIC" | Journal of Rheumatology. Vol. 11, Pg. 843, 1984. |
Consensus Reports:
IARC Cancer Review: Group 1 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 13 (1977),p. 39.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) .
SAFETY PROFILE:
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. A deadly human poison by an unspecified route. An experimental poison by intramuscular route. Moderately toxic by subcutaneous and intravenous routes. Human systemic effects: nausea or vomiting, cholestatic jaundice, and eye effects. An experimental teratogen. Other experimental reproductive effects. See also GOLD COMPOUNDS. When heated to decomposition it emits very toxic fumes of SOx.
Hazard Codes:
 Xn
Xn
Risk Statements:
R42/43:May cause sensitization by inhalation and skin contact.
Safety Statements:
S22:Do not breathe dust.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: 2811
HazardClass: 6.1(b)
PackingGroup: III
Standards and Recommendations:
Analytical Methods:
Related Searches
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione