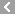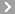CAS No.:61-75-6
Name: Bretylium tosilate
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intramuscular | 100mg/kg (100mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| dog | LD50 | intravenous | 43mg/kg (43mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| mouse | LD50 | intramuscular | 64mg/kg (64mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| mouse | LD50 | intraperitoneal | 39mg/kg (39mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 155, Pg. 69, 1965. | |
| mouse | LD50 | intravenous | 14mg/kg (14mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| mouse | LD50 | oral | 400mg/kg (400mg/kg) | British Journal of Pharmacology and Chemotherapy. Vol. 14, Pg. 536, 1959. | |
| mouse | LD50 | subcutaneous | 72mg/kg (72mg/kg) | British Journal of Pharmacology and Chemotherapy. Vol. 14, Pg. 536, 1959. | |
| rabbit | LD50 | intramuscular | 160mg/kg (160mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| rabbit | LD50 | intravenous | 30mg/kg (30mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| rat | LD50 | intramuscular | 84mg/kg (84mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| rat | LD50 | intraperitoneal | 57mg/kg (57mg/kg) | Pharmacology: International Journal of Experimental and Clinical Pharmacology. Vol. 21, Pg. 256, 1980. | |
| rat | LD50 | intravenous | 17mg/kg (17mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 148, 1979. | |
| women | TDLo | intravenous | 81500ug/kg/15 (81.5mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: COMA VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION |
American Journal of Emergency Medicine. Vol. 13, Pg. 177, 1995. |
Consensus Reports:
SAFETY PROFILE:
A poison by ingestion, intraperitoneal, subcutaneous, intravenous, and intramuscular routes. An anti-adrenergic agent and antiarrhythmic cardiac depressant. When heated to decomposition it emits very toxic fumes of SOx, NH3, NOx, and Br−. See also SULFONATES.
Hazard Codes  Xn
Xn
Risk Statements 20/21/22
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements 36
S36:Wear suitable protective clothing.
RTECS BO9450000
Standards and Recommendations:
Analytical Methods:
Related Searches
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione