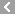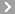CAS No.:42609-52-9
Name: Daimuron
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | skin | > 3500mg/kg (3500mg/kg) | Japan Pesticide Information. Vol. (40), Pg. 32, 1982. | |
| mouse | LDLo | intraperitoneal | > 3500mg/kg (3500mg/kg) | Japan Pesticide Information. Vol. (36), Pg. 40, 1979. | |
| mouse | LDLo | oral | > 6500mg/kg (6500mg/kg) | Japan Pesticide Information. Vol. (36), Pg. 40, 1979. | |
| mouse | LDLo | subcutaneous | > 3500mg/kg (3500mg/kg) | Japan Pesticide Information. Vol. (36), Pg. 40, 1979. | |
| rat | LD50 | skin | > 3500mg/kg (3500mg/kg) | Japan Pesticide Information. Vol. (40), Pg. 32, 1982. | |
| rat | LDLo | intraperitoneal | > 3500mg/kg (3500mg/kg) | Japan Pesticide Information. Vol. (36), Pg. 40, 1979. | |
| rat | LDLo | oral | > 4gm/kg (4000mg/kg) | Japan Pesticide Information. Vol. (36), Pg. 40, 1979. | |
| rat | LDLo | subcutaneous | > 3500mg/kg (3500mg/kg) | Japan Pesticide Information. Vol. (36), Pg. 40, 1979. |
Consensus Reports:
SAFETY PROFILE:
Mildly toxic by ingestion and subcutaneous routes. When heated to decomposition it emits toxic fumes of NOx.
Standards and Recommendations:
Analytical Methods:
P-urea method
P-urea preparation of sodium cyanate with the reaction of p-toluidine hydrochloride solution to control the reaction temperature is 30°C, a little too much sodium cyanate, urea obtained for toluene. Urea, sodium cyanate can be made with calcined sodium carbonate.
Preparation of chloro-isopropylbenzene 2 - phenyl-propene in acetonitrile solvent with hydrogen chloride addition, the introduction of chlorine atom side chain to produce chlorinated cumene.
Herbicide-long synthesis of p-cumene role of urea and chloride, synthetic herbicide lung. Join toluene urea 0.15mol, chloride, cumene 0.1mol, α-methyl styrene 48g and acetonitrile 160g, at 40 °C stirring reaction 6h, put it aside at room temperature, separation of crystals 32g, yield 80%.
α, α-dimethyl benzyl isocyanate France
Law is the α, α-2-methyl chloride in non-aqueous organic solvents and metal chloride catalysts in the presence of sodium cyanate reacted with α, α-dimethyl benzyl isocyanate, filtered to remove sodium chloride by-product , and then with the addition of p-toluidine obtained a good yield of herbicide-lung. Such as: in the reactor by adding ethyl acetate 50mL, 90% sodium cyanate 6.5g (90mmol), zinc chloride (0.5mol), pyridine (1mmol), stirring heated to 50°C, and then join the α, α-2 Methyl chloride 7.73g (50mmol) at 50 °C reaction 1h. Obtained α, α-dimethyl benzyl isocyanate. α, α-dimethyl benzyl isocyanate, and p-toluidine in toluene or chlorobenzene in addition, the almost quantitative yield of herbicide-lung.
Related Searches
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione