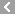CAS No.:50-91-9
Name: Floxuridine
Details Introduction
TOXICITY DATA with REFERENCE:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | TDLo | intravenous | 5mg/kg/14D-C (5mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" GASTROINTESTINAL: NAUSEA OR VOMITING GASTROINTESTINAL: OTHER CHANGES |
Cancer Vol. 34, Pg. 972, 1974. |
| mouse | LD50 | intraperitoneal | 650mg/kg (650mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" GASTROINTESTINAL: NAUSEA OR VOMITING |
Proceedings of the Society for Experimental Biology and Medicine. Vol. 97, Pg. 470, 1958. |
| mouse | LD50 | oral | 147mg/kg (147mg/kg) | Progress Report for Contract No. NIH-NCI-E-C-72-3252, Submitted to the National Cancer Institute by Litton Bionetics, Inc. Vol. NCI-E-C-72-3252, Pg. 1973, | |
| mouse | LD50 | unreported | 550mg/kg (550mg/kg) | Cancer Research. Vol. 46, Pg. 2703, 1986. | |
| rat | LD50 | intraperitoneal | 1600mg/kg (1600mg/kg) | Clinical Proceedings of the Children's Hospital of the District of Columbia. Vol. 18, Pg. 307, 1962. | |
| rat | LD50 | oral | 215mg/kg (215mg/kg) | Progress Report for Contract No. NIH-NCI-E-C-72-3252, Submitted to the National Cancer Institute by Litton Bionetics, Inc. Vol. NCI-E-C-72-3252, Pg. 1973, | |
| women | TDLo | parenteral | 173mg/kg/82W- (173mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" | Journal of the National Medical Association. Vol. 79, Pg. 669, 1987. |
Consensus Reports:
EPA Genetic Toxicology Program.
SAFETY PROFILE:
Hazard Codes 2'-Deoxy-5-fluorouridine (CAS NO.50-91-9):  Xn,
Xn, T,
T, Xi
Xi
Risk Statements: 68-36/37/38-40-20/21/22
R68: Possible risk of irreversible effects.
R36/37/38: Irritating to eyes, respiratory system and skin.
R40: Limited evidence of a carcinogenic effect.
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 22-36/37/39-26
S22: Do not breathe dust.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
RIDADR: UN 2811 6.1/PG 3
WGK Germany: 3
RTECS: YU7525000
F: 10-23
Hazard Note: Toxic
HazardClass: 6.1
PackingGroup: III
Poison by ingestion. Moderately toxic by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. Human systemic effects: hypermotility, diarrhea, nausea, vomiting and other gastrointestinal effects, allergic dermatitis, and bone marrow changes. Human mutation data reported. When heated to decomposition it emits very toxic fumes of F− and NOx .
Standards and Recommendations:
Analytical Methods:
Related Searches

Related Suppliers
- Simagchem Corporation
- Dayang Chem (Hangzhou) Co.,Ltd.
- ORCHID CHEMICAL SUPPLIES LTD
- Wuhan Fortuna Chemical Co.,Ltd
- Xiamen Hisunny Chemical Co.,Ltd
- dalian kenost Trade Co., LTD
- Shaanxi King Stone Enterprise Company Limited
- SHAANXI TOP PHARM CHEMICAL CO.LTD
- hangzhou neway chemicals co., ltd.
- Chemieliva Pharmaceutical Co., Ltd.
- NANJING BOLD CHEMICAL CO.,LTD
- Active Pharmaceutical Ingredients
- rxn chemicals pvt ltd
- HAORUI ENTERPRISES LLC
- CHEMIMPEX INT'L INC
- Hongene Biotechnology Limited
- Tosta Enterprise Co., Ltd.
- Jinan Great Chemical Co.,Ltd.
- Changzhou Da ou Chemical. Co.ltd
- Tocopharm Co., Ltd.
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione