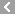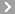CAS No.:156-51-4
Name: beta-Phenylethylhydrazine sulfate
Details Introduction
TOXICITY DATA with REFERENCE:
| 1. |
mma-sat 800 µg/plate |
NEZAAQ Nippon Eiseigaku Zasshi. Japanese Journal of Hygiene. 33 (1978),474. | ||
| 2. |
dnr-esc 4300 nmol/plate |
JTEHD6 Journal of Toxicology and Environmental Health. 9 (1982),287. | ||
| 3. |
orl-mus TDLo:28 g/kg/77W-C:NEO |
CNREA8 Cancer Research. 36 (1976),917. | ||
| 4. |
orl-hmn TDLo:1070 µg/kg/D:CNS,GIT,CVS |
34ZIAG Toxicology of Drugs and Chemicals ,Deichmann, W.B.,New York, NY.: Academic Press, Inc.,1969,461. | ||
| 5. |
orl-man TDLo:46 mg/kg/10W-I:LIV, |
AJMEAZ American Journal of Medicine. 80 (1986),689. | ||
| 6. |
orl-wmn TDLo:18 mg/kg |
ANASAB Anaesthesia. 41 (1986),53. | ||
| 7. |
orl-rat LD50:210 mg/kg |
ANYAA9 Annals of the New York Academy of Sciences. 107 (1963),899. | ||
| 8. |
orl-mus LD50:156 mg/kg |
ANYAA9 Annals of the New York Academy of Sciences. 107 (1963),899. | ||
| 9. |
ipr-mus LD50:162 mg/kg |
JMCMAR Journal of Medicinal Chemistry. 18 (1975),20. | ||
| 10. |
scu-mus LD50:125 mg/kg |
JOENAK Journal of Endocrinology. 30 (1964),205. | ||
Consensus Reports:
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 312.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 ,1980,p. 175.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 ,1980,p. 175.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . EPA Genetic Toxicology Program.
SAFETY PROFILE:
Poison by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Human systemic effects by ingestion: wakefulness, blood pressure lowering, constipation, hepatitis, fibrous hepatitis. Experimental reproductive effects. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. Used as a drug for the treatment of depression. When heated to decomposition it emits very toxic fumes of SOx and NOx.
Hazard Codes  Xn
Xn
Risk Statements 22
R22:Harmful if swallowed.
RIDADR UN 2811 6.1/PG 3
WGK Germany 3
RTECS MV8750000
HazardClass 6.1(b)
PackingGroup III
Standards and Recommendations:
Analytical Methods:
Related Searches
Other Product
- -
- -
- -
- -
- -
- -
- -
- -
- -
- (4S,6R)-6-{2-[(2-hydroxy-ethyl)-(4-methoxy-benzenesulfonyl)-amino]-ethoxy}-4-isopropyl-5,6-dihydro-4H-pyran-2-carboxylic acid allyl ester
- [1,4'-Bipiperidine]-1'-carboxylic acid, 4-(1H-indol-7-yl)-, ethyl ester
- 1,3,5-Tris(4-acetamidophenyl)penta-1,5-dione
- 1,5-Bis(3-aminophenyl)-3-(4-N,N-di-methylaminophenyl)penta-1,5-dione
- 1,5-Bis(4-acetamidophenyl)-3-phenylpenta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-(4-acetamido-phenyl)penta-1,5-dione
- 1,5-Bis(4-aminophenyl)-3-phenylpenta-1,5-dione